Title
Enhanced Control of Hypertension and Thrombectomy Stroke Study
Short title
The ENCHANTED-MT trial
Study phase
III
Trial registration
ClinicalTrials.gov Identifier:
NCT04140110
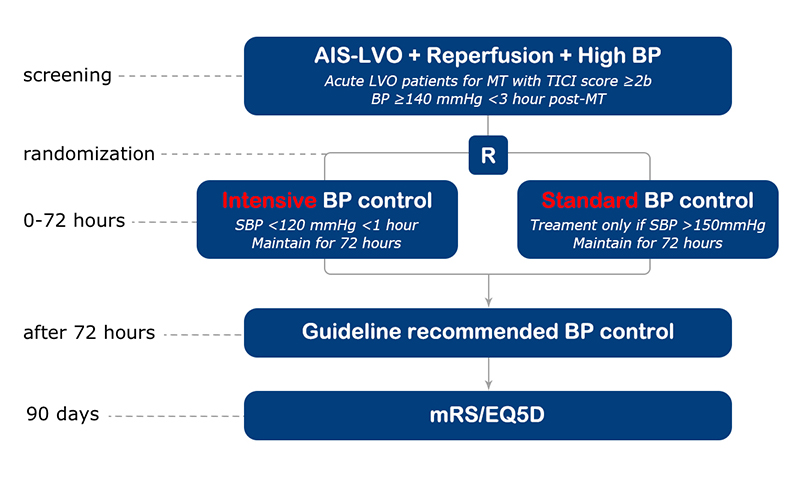
Study design
Investigator-initiated, international, multicentre, prospective, randomised, open, blinded end-point assessed (PROBE) clinical trial.
Objectives
To determine the effectiveness of more intensive blood pressure (BP) lowering target (
<120 mmHg) as compared to a higher BP management target (140-180mmHg) on functional outcome in patients who have had successful recanalization with mechanical thrombectomy (MT) for acute ischemic stroke (AIS) due to large vessel occlusion (LVO).
Inclusion Criteria
- Age ≥18 years;
- Diagnosis of AIS with LVO confirmed by brain imaging;
- To receive MT
<24 hours after AIS onset according to local guidelines;
- Successful recanalization (TICI score ≥2b) after MT;
- Sustained systolic BP ≥140 mmHg (defined as 2 successive readings
<10 mins) within 3 hours after recanalization;
- Provide written informed consent (or approved surrogate).
Exclusion Criteria
Patients will NOT be eligible if there is one or more of the following;
- Unlikely to potentially benefit from therapy (e.g. advanced dementia) or very high likelihood of death within 24 hours post-MT, judged by responsible treating clinician;
- Other medical illness that interferes with outcome assessments and follow-up (e.g. known significant pre-stroke disability (mRS scores 3-5), advance cancer and renal failure);
- Definite indication/contraindication to different intensities of BP lowering treatment;
- Specific contraindications to any of the BP agents to be used (eg, patients who are hypersensitive (allergic) to any of the ingredients);
- Patients with aortic isthmus stenosis and arteriovenous shunt (exception: patients with haemodynamically inactive dialysis shunt);
- Women who are lactating;
- Currently participating in another trial which would interfere with outcome assessments.
Number of Planed Participants
2257 patients
Primary outcome
functional recovery, defined as a shift (improvement) in scores on the modified Rankin scale (mRS) at 90 days.
Secondary outcomes
any intracranial haemorrhage (ICH), symptomatic intracerebral haemorrhage (sICH), early neurological deterioration, imaging assessment (e.g. infarct size, edema volume), death, disability, HRQoL, duration of hospitalization, residence; and health service use for calculation of resources and costs.
Study procedure
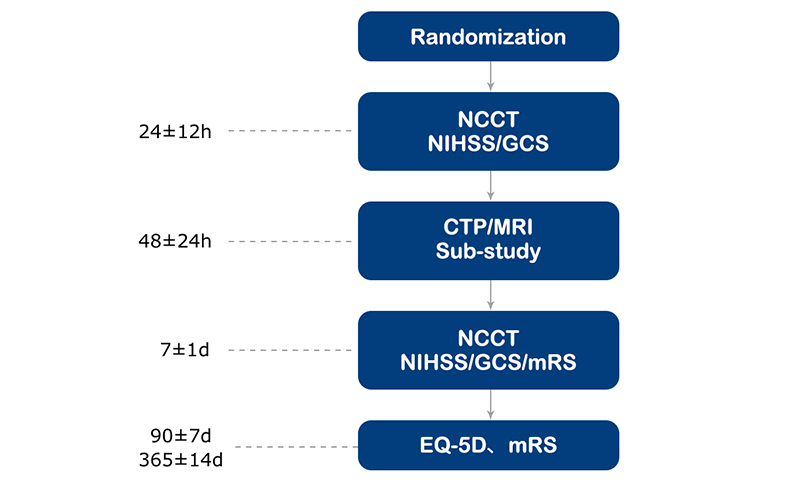
Follow-up procedure